Protons 7 Neutrons 8 Electrons 10
Inside a nucleus a proton can transform into a neutron via inverse beta decay if an energetically allowed quantum state. Some atoms of nitrogen however have eight electrons.
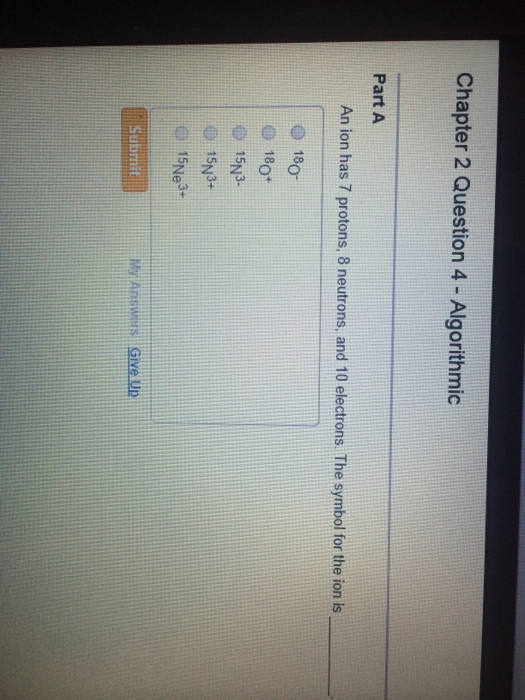
Solved An Ion Has 7 Protons 8 Neutrons And 10 Electrons Chegg Com
Calculating Protons Electrons and Neutrons.

. This means a neutral atom of nitrogen must have seven electrons to match its seven protons. In a neutral atom the number of protons must equal the number of electrons. Finding the number of protons neutrons and electrons in a given element isnt as hard as it sounds.
A good video explaining atomic structure molecules formation. Discovery of Protons and Neutrons Discovery of Protons The discovery of protons dates back to the year 1815 when the English chemist William Prout suggested that all atoms are made up of hydrogen atoms which he referred to as protyles. Once you know where to look finding the number of protons neutrons and electrons will be a breeze.
One example of this decay is carbon-14 6 protons 8 neutrons that decays to nitrogen-14 7 protons 7 neutrons with a half-life of about 5730 years. Part 1 of 2. They are atoms of N-15 an isotope of nitrogen.
Many nitrogen atoms have seven neutrons 14-7 7. Oftentimes part of your answer will be right in front of you in the periodic table.

How Many Protons Neutrons And Electrons Does The Atom Above Have 12 Protons 7 Neutrons 1 Brainly Com

First 20 Elements Storyboard By Oliversmith

What Is The Mass Number Of An Atom With 7 Protons 8 Neutrons And 7 Electrons Brainly Com
No comments for "Protons 7 Neutrons 8 Electrons 10"
Post a Comment